News
Sementis raises $5m for COVID vaccine as Hickinbotham takes majority stake
Adelaide biotechnology company Sementis Limited has raised more than $5.2 million to accelerate work on its next- generation...
Researchers in Australia have developed a coronavirus disease 2019 (COVID-9) vaccine that induced robust and long-lived humoral and cellular immune responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in both young and aging mice.
The team used the vaccinia virus-based Sementis Copenhagen Vector (SCV) to develop a vaccine encoding the SARS-CoV-2 spike glycoprotein that mediates viral attachment and entry into host cells. This spike protein is a primary target of host immune responses following natural SARS-CoV-2 infection or vaccination.
Vaccinated mice rapidly developed robust spike-specific CD8 T cells and neutralizing antibody responses that significantly increased following a second dose.
Neutralizing antibody activity was maintained for up to nine months post-vaccination, and durable immune memory was evident.
The team – from the University of South Australia in Adelaide and Sementis Limited in Hackney – says the study data support the progression of SCV-based COVID-19 vaccine candidates towards efficacy testing in preclinical SARS-CoV-2 infectious disease challenge trials.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
The challenges faced in COVID-19 vaccine design
Since the COVID-19 outbreak first began in Wuhan, China, in late December 2019, the causative agent – SARS-CoV-2 – has infected more than 222 million people and claimed the lives of more than 4.59 million.
Fatality rates are disproportionately high in older individuals and those with co-morbidities such as diabetes, cardiovascular disease and immunosuppression.
The rapid surge in cases during the second and third waves of infection across several countries has been attributed to the emergence of variants of concern that exhibit increased transmissibility and immune escape owing to mutations acquired in the viral genome, particularly the spike glycoprotein.
Most of the vaccines undergoing development, including those currently approved, exclusively target this spike protein to generate neutralizing antibodies and confer protection.
However, due to the unprecedented speed of this vaccine development, knowledge gaps remain regarding aspects such as the duration of protection and the induction and relative importance of cell-mediated immunity.
Furthermore, vaccines must meet the requirements for rapid and successful deployment within the context of mass vaccination programs, with specific considerations given to large-scale manufacture and distribution logistics.
How can the Sementis Copenhagen Vector help?
The Sementis Copenhagen Vector (SCV) is a viral vector technology that builds on the favorable characteristics of the parental vaccinia virus (VACV) vector. These characteristics include long-lasting immune responses, significant antigen payload capacity, and cold-chain-independent distribution.
“Uniquely for a VACV-based vector, it has also been paired with a proprietary manufacturing cell line to generate a platform system that can facilitate large scale and facile manufacturability of all SCV-based vaccines,” says Preethi Eldi and colleagues.
“The efficacy of SCV vaccines has also been established in mouse models of disease and non-human primate immunogenicity and infectious disease challenge studies,” adds the team.
What did the current study involve?
The researchers constructed a first-generation SCV vaccine encoding the full-length, native SARS-CoV-2 spike glycoprotein (SCV-S) from the original Wuhan isolate.
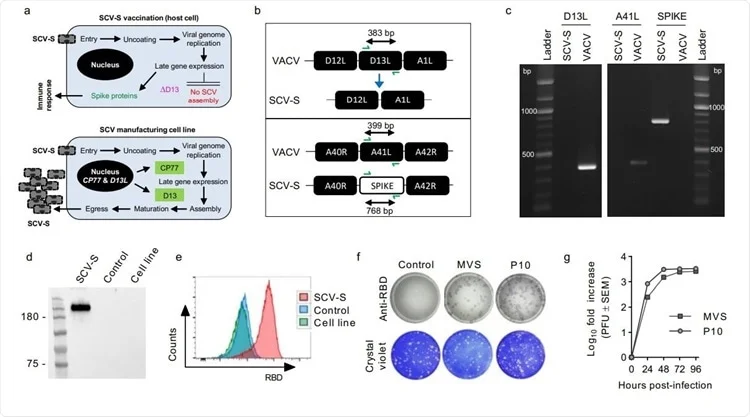
The SCV-S was tested for its ability to induce a spike-specific cellular immune response in C57BL/6J mice.
Immunogenicity studies showed that vaccinated mice rapidly developed spike-specific polyfunctional CD8 T cell responses among both young and aging mice in just one week.
Vaccination also induced robust levels of circulating spike-specific antibodies, with significant neutralizing activity detected within two weeks.
Importantly, following a booster dose of the vaccine, circulating neutralizing antibody levels were maintained, without any discernible decay, over a nine-month period in both the young and old mice.
“The sustained circulating antibody levels are encouraging, particularly in light of recent studies suggesting a decay in neutralization activity in vaccinated and convalescent patients,” writes Eldi and the team.
n addition, assessment of long-lived CD8 T cell and antibody-secreting cell compartments revealed the induction of durable spike-specific immune memory following vaccination in both age groups.
Furthermore, spike-specific antibodies contained neutralizing activity against the alpha (B.1.1.7) and beta (B.1.351) variants of concern that have previously been shown to exhibit increased transmissibility (B.1.1.7) and enhanced immune escape (B.1.351).
What did the authors conclude?
The researchers say the data support the progression of SCV-based COVID-19 vaccine candidates towards authentic efficacy testing in recognized preclinical SARS-CoV-2 infectious disease challenge trials and clinical development programs.
“The encouraging immunogenicity profile with a single SARS-CoV-2 antigen also provides impetus to include additional SARS-CoV-2 antigens in second-generation SCV COVID-19 vaccines,” says Eldi and colleagues.
This would “broaden and strengthen vaccine immunogenicity profiles for enhanced vaccine protective efficacy against current VOCs [variants of concern] and new emerging variants as they inevitably become established within the affected global population,” concludes the team.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
Eldi P, et al. The vaccinia-based Sementis Copenhagen Vector COVID-19 vaccine induces broad and durable cellular and humoral immune responses. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.09.06.459206, https://www.biorxiv.org/content/10.1101/2021.09.06.459206v1